分子の空間的構造の精密な把握に基づく有機構造材料の開発
メンバー: 岡本昭子、米澤宣行
分野: 基礎化学、材料化学
所属: 工学研究院
キーワード: 非共平面性芳香環集積分子、 空間的構成、 精密分子設計、 X線結晶構造解析、 動的NMR解析
ウェブサイト:
研究概要
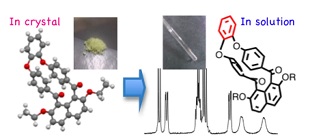
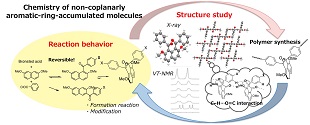
現行の高強度・高性能性材料の主要成分である芳香族単位が集積したり他の構築部位と関わるときに形成する相互作用が、その材料の物性や機能の発現に大きく影響していることは明らかです。しかし、これまで材料化学分野における相互作用解析研究はπ…π相互作用に限定したものがほとんどでした。私たちは「芳香環が同一平面上に並ばないで非共平面的に配置した分子」(peri-アロイルナフタレン)を解析モデルに用いて、X線結晶構造解析を駆使して網羅的に収集した知見を基に、「π…π相互作用以外」の、芳香環が関与する非共有結合性相互作用の種類と起源を正確に把握し、有機固体における各相互作用の相対的な寄与の大きさと形成要件を解明することに取り組んでいます。また、精密な分子修飾により合成した、「芳香環の配置が制限された」分子群の、固体中、溶液中での空間構造の特徴を系統的に整理することを通して、異なる次元における芳香環の空間的構成の安定化要素、変換挙動の精確な理解と解釈に繋げています。将来的には、ユニークな芳香環配置分子を構築単位とする超高性能性有機構造材料の開発に応用したいと考えています。
主要論文・参考事項
1) S. Yoshiwaka, K. Ogata, N. Yonezawa and A.Okamoto*, “Molecular structure of bridged peri-aroylnaphthalene compound having cyclohexane-cis-1,2-dioxy hinge moiety in solution”, European Chemical Bulletin, 2015, 4(4), 195-201.
2) S. Yoshiwaka, D. Hijikata, N. Yonezawa and A.Okamoto*, "Solution structures of bridged peri-aroylnaphthalene compounds having 1,2- or 1,3-benzenedioxy-hinge moiety",
European Chemical Bulletin, 2015, 4(4), 202–206.
3) S. Mohri, S. Ohisa, K. Isozaki, N. Yonezawa and A. Okamoto*,
“Hydrogen bonding between fluoro group and aromatic hydrogen leading stripe structure of R-isomeric column and S-isomeric one: Crystal structure of 2,7-dimethoxynaphthalen-1-yl(3-fluorophenyl)methanone and a comparison with its 1-aroylnaphthalene analogues”, Acta Cryst. Section C (Structure Chemistry), 2015, C71, 344–350.
4) A. Okamoto*, S. Ohisa, S. Yoshiwaka and N. Yonezawa, “Conformational exchange of 1,8-dibenzoyl-2,7-dimethoxynaphthalene analogues in solution”, European Chemical Bulletin, 2015, 4(2), 67-73.
5) S. Yoshiwaka, S. Ohisa, N. Yonezawa and A.Okamoto*, “Synthesis and crystal structure of bridged peri-aroylnaphthalene derivatives”, European Chemical Bulletin, 2014, 3(12), 1142–1147.
6) Akiko Okamoto and Noriyuki Yonezawa, “Unique and Specific Reaction Behavior and Characteristic Spatial Organization of Non-coplanarly Aromatic-ring-accumulated Molecular Compounds”, Journal of Synthetic Organic Chemistry, Japan, 2015, 73(4), 339–360.
お問い合わせ先
東京農工大学・先端産学連携研究推進センター
urac[at]ml.tuat.ac.jp([at]を@に変換してください)
Study on spatial organization of non-coplanarly accumulated-aromatic rings molecules aiming organic structure materials

Research members: Dr. Akiko Okamoto, Dr. Noriyuki Yonezawa
Research fields: Basic chemistry, Materials chemistry
Departments: Institute of Engineering
Keywords: Non-coplanarly accumulated-aromatic rings molecules, Spatial organization, Molecular design, X-ray crystallography, Dynamic NMR study
Web site:
Summary

Molecules of congested non-coplanarly-accumulated aromatic rings structure such as binaphthyl and biphenyl have attracted the interests of the chemists in organic chemistry and material chemistry fields as unique building blocks affording characteristic optic and electronic properties in addition to construction of chiral molecular scaffold for organic synthesis. To integrate information about stabilization factors of crystalline accumulation state of organic molecules is significantly important as fundamental study for development of such functional organic materials.
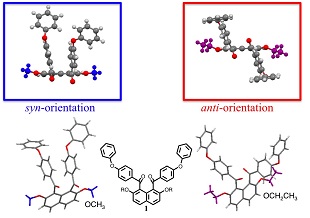
Recently, the authors have reported peri(1,8)-selective diaroylation of naphthalene derivatives. According to the X-ray crystal structure analyses of the resulting 1,8-diaroylnaphthalene compounds, they have been proved to have characteristic structure of non-coplanarly aromatic-rings-accumulated structures. As single molecular structure, two aroyl groups are attached to the naphthalene ring in an almost perpendicular fashion and oriented in an opposite direction. The highly congested circumstance leading non-coplanar spatial organization of aromatic rings accumulation of these molecules means the reduced development of conjugation inevitably resulting in relatively lower contribution of interaction originated from conjugated planes. Instead, it affords the opportunity to reveal the normally hidden interactions. The authors have intended to disclose the stabilization factors of non-coplanarly aromatic-rings-accumulated compounds through investigation of the spatial organization of single molecule, the molecular accumulation structure, and the non-bonding molecular interactions in 1,8-diaroylnaphthalene homologues. In the course of these studies, the authors have designed and synthesized 1,8-diaroylated-2,7-dialkoxynaphthalene compounds followed by determination of the crystal structures, e.g., 1,8-bis(4-fluorobenzoyl)-2,7-dimethoxynaphthalene, 2,7-diethoxy-1,8-bis(4-fluorobenzoyl)naphthalene, 2,7-dibutoxy-1,8-bis(4-fluorobenzoyl)naphthalene, and 1,8-bis(4-fluorobenzoyl)-2,7-diphenoxynaphthalene. In this study, the authors will report crystal structure of peri-aroylnaphthalene copounds and discuss the contribution of the non-bonding molecular interactions to stabilization of the molecular packing through comparison of the crystal structures of the homologues in detail.
Reference articles and patents
1) S. Yoshiwaka, K. Ogata, N. Yonezawa and A.Okamoto*, “Molecular structure of bridged peri-aroylnaphthalene compound having cyclohexane-cis-1,2-dioxy hinge moiety in solution”, European Chemical Bulletin, 2015, 4(4), 195-201.
2) S. Yoshiwaka, D. Hijikata, N. Yonezawa and A.Okamoto*, "Solution structures of bridged peri-aroylnaphthalene compounds having 1,2- or 1,3-benzenedioxy-hinge moiety",
European Chemical Bulletin, 2015, 4(4), 202–206.
3) S. Mohri, S. Ohisa, K. Isozaki, N. Yonezawa and A. Okamoto*,
“Hydrogen bonding between fluoro group and aromatic hydrogen leading stripe structure of R-isomeric column and S-isomeric one: Crystal structure of 2,7-dimethoxynaphthalen-1-yl(3-fluorophenyl)methanone and a comparison with its 1-aroylnaphthalene analogues”, Acta Cryst. Section C (Structure Chemistry), 2015, C71, 344–350.
4) A. Okamoto*, S. Ohisa, S. Yoshiwaka and N. Yonezawa, “Conformational exchange of 1,8-dibenzoyl-2,7-dimethoxynaphthalene analogues in solution”, European Chemical Bulletin, 2015, 4(2), 67-73.
5) S. Yoshiwaka, S. Ohisa, N. Yonezawa and A.Okamoto*, “Synthesis and crystal structure of bridged peri-aroylnaphthalene derivatives”, European Chemical Bulletin, 2014, 3(12), 1142–1147.
6) Akiko Okamoto and Noriyuki Yonezawa, “Unique and Specific Reaction Behavior and Characteristic Spatial Organization of Non-coplanarly Aromatic-ring-accumulated Molecular Compounds”, Journal of Synthetic Organic Chemistry, Japan, 2015, 73(4), 339–360.
Contact
University Research Administration Center(URAC),
Tokyo University of Agriculture andTechnology
urac[at]ml.tuat.ac.jp
(Please replace [at] with @.)