ヒドリド転位を鍵とする炭素ー水素結合変換型環化反応の開発
メンバー: 森啓二
分野: 基礎化学
所属: 工学研究院
キーワード: 酸化還元、 炭素ー水素結合官能基化、 環化反応
ウェブサイト:
研究概要
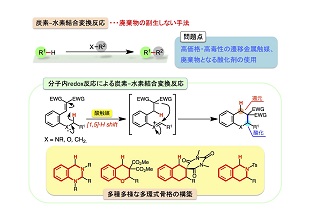
医薬・農薬など有用な中間体であるファインケミカルズの合成には、数多くの行程を必要とします。当然ながらその過程においては、多量の廃棄物の副生を余儀なくされます。近代社会で環境問題が大きく取りざたされることとも相まって、近年、環境調和型プロセスへの需要が急速に高まっています。様々な化合物中に偏在する炭素−水素結合を直接変換する反応(C–H結合官能基化反応)は、その要件を満たす手法として近年大きな注目を集めています。その有用性からこれまでに様々な手法が開発されてきましたが、最も不活性なC(sp3)–H結合の変換は、有機合成手法の洗練された現在でもなお、困難な課題です。また従来法では、高活性・毒性のある遷移金属触媒や、反応後に廃棄物となる酸化剤の使用を要するため、更なる低環境負荷型変換法の開拓が強く望まれていました。
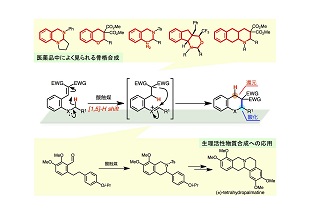
近年我々の研究グループではこの課題の解決を目指し研究に取り組んだ結果、ヒドリド転位を介する分子内での酸化還元を利用した、遷移金属や酸化剤を必要としない合成手法(「分子内redox型環化反応」)の開発に成功しました。本手法は(1)不活性なC(sp3)–H結合の直接的変換、(2)単純な酸(Brønsted酸やLewis酸)による触媒反応、(3)機能性物質の主要骨格である多環式化合物の合成、という多くの特徴を併せ持つ魅力的な反応系です。最近は、この反応を起点とした複雑な多環性骨格の構築法や生理活性化合物の合成を目指し、日々研究に取り組んでいます。
主要論文・参考事項
(1) Synthesis of 3-Aryl-1-trifluoromethyltetrahydroisoquinolines by Brønsted Acid Catalyzed C(sp3)–H Bond Functionalization, Keiji Mori, Nobuaki Umehara, Takahiko Akiyama,
Adv. Synth. Catal. 2015, 357, 901 (Selected as VIP and Front cover article).
(2) Double C(sp3)-H Bond Functionalization Mediated by Sequential Hydride Shift/Cyclization Process: Diastereoselective Construction of Polyheterocycles, Keiji Mori, Kazuki Kurihara, Shinnosuke Yabe, Masahiro Yamanaka, Takahiko Akiyama,
J. Am. Chem. Soc. 2014, 136, 3744.
(3) Concise Route to 3-Arylisoquinoline Skeleton by Lewis Acid Catalyzed C(sp3)–H Bond Functionalization and Its Application to Formal Synthesis of (±)-Tetrahydropalmatine, Keiji Mori, Taro Kawasaki, Takahiko Akiyama,
Org. Lett. 2012, 14, 1436.
(4) Selective Activation of Enantiotopic C(sp3)–Hydrogen by Means of Chiral Phosphoric Acid: Asymmetric Synthesis of Tetrahydroquinoline Derivatives, Keiji Mori, Kensuke Ehara, Kazuki Kurihara, Takahiko Akiyama,
J. Am. Chem. Soc. 2011, 133, 6166 (Highlighted in Synfacts).
(5) Expeditious Construction of a Carbobicyclic Skeleton via a sp3-C–H Functionalization: Hydride Shift from an Aliphatic Tertiary Position in an Internal Redox Process, Keiji Mori, Shosaku Sueoka, Takahiko Akiyama,
J. Am. Chem. Soc. 2011, 133, 2424.
お問い合わせ先
東京農工大学・先端産学連携研究推進センター
urac[at]ml.tuat.ac.jp([at]を@に変換してください)
C(sp3)–H Bond Functionalization Mediated by Hydride Shift/Cyclization System

Research members: Keiji Mori PhD.
Research fields: Basic chemistry
Departments: Institute of Engineering
Keywords: redox system, C–H bond functionalization, cyclization
Web site:
Summary

The multi-step sequence was required for the synthesis of fine chemicals (such as drugs, agrochemicals). Substantial amount of organic (inorganic) wastes were produced in the process. Thus, the development of novel and environmentally benign process was strongly demanded. The C–H bond functionalization reaction is the highly promising approach to satisfy this demands. The transformation of inert C(sp3)–H bond was difficult task, even though the extensive studies for the C–H bond functionalization reaction have been conducted. Furthermore, the employment of the toxic and expensive transition metal and excess amount of external oxidants was required to accomplish this transformation in most cases.
Recently, we have developed novel C(sp3)–H bond functionalization strategy, hydride shift/cyclization system (internal redox system). There are three key features in the reaction: (1) direct transformation of inert C(sp3)–H bond, (2) catalytic reaction with simple (conventional) acid (Brønsted or Lewis acids), and (3) construction of fused-polycyclic skeleton by single operation. Our future plan is the synthesis of complicated polycyclic framework and some biologically active compounds based on this method.
Reference articles and patents
(1) Synthesis of 3-Aryl-1-trifluoromethyltetrahydroisoquinolines by Brønsted Acid Catalyzed C(sp3)–H Bond Functionalization, Keiji Mori, Nobuaki Umehara, Takahiko Akiyama,
Adv. Synth. Catal. 2015, 357, 901 (Selected as VIP and Front cover article).
(2) Double C(sp3)-H Bond Functionalization Mediated by Sequential Hydride Shift/Cyclization Process: Diastereoselective Construction of Polyheterocycles, Keiji Mori, Kazuki Kurihara, Shinnosuke Yabe, Masahiro Yamanaka, Takahiko Akiyama,
J. Am. Chem. Soc. 2014, 136, 3744.
(3) Concise Route to 3-Arylisoquinoline Skeleton by Lewis Acid Catalyzed C(sp3)–H Bond Functionalization and Its Application to Formal Synthesis of (±)-Tetrahydropalmatine, Keiji Mori, Taro Kawasaki, Takahiko Akiyama,
Org. Lett. 2012, 14, 1436.
(4) Selective Activation of Enantiotopic C(sp3)–Hydrogen by Means of Chiral Phosphoric Acid: Asymmetric Synthesis of Tetrahydroquinoline Derivatives, Keiji Mori, Kensuke Ehara, Kazuki Kurihara, Takahiko Akiyama,
J. Am. Chem. Soc. 2011, 133, 6166 (Highlighted in Synfacts).
(5) Expeditious Construction of a Carbobicyclic Skeleton via a sp3-C–H Functionalization: Hydride Shift from an Aliphatic Tertiary Position in an Internal Redox Process, Keiji Mori, Shosaku Sueoka, Takahiko Akiyama,
J. Am. Chem. Soc. 2011, 133, 2424.
Contact
University Research Administration Center(URAC),
Tokyo University of Agriculture andTechnology
urac[at]ml.tuat.ac.jp
(Please replace [at] with @.)

